Which Definition Best Describes the Term Molar Mass
Which definition best describes the term molar mass. Which definition best describes the term molar mass.

Solved Which Definition Best Describes The Term Molar Mass Chegg Com
Molar mass is defined as the mass in grams of one mole of a substance.

. Molar mass is the mass of a given substance divided by its amount of substance. Which definition best describes the term molar mass. Which definition best describes the term molar mass.
A mole is a numerical unitspecifically it represents the number of carbon atoms in a 1200 g mass of 12C and this number is N A Avogadros constant 602214085774 1023 mol1. Molar mass is defined as the mass in grams of one mole of a substance. Which definition best describes the term molar mass.
The mass in grams of one mole of a substance Isotopes are atoms of a particular element that contain a different number of. The unit in which molecular mass is measured is amu. The amount of molecules or atoms or compounds present in one mole of substance is given by this.
Atoms Carbon Isotope Molar Mass Mole Molecular Weight. The molar mass is obtained by dividing the mass of a particular substance by the amount of it. Mathematically Molar mass For example molar mass of 1 mole of molecule will be calculated as follows.
Calculate the molar mass of each compound given below. The molar mass is the mass of a given chemical element or chemical compound g divided by the amount of substance mol. Molecular mass differs because of the isotopes.
It is usually denoted by the symbol M. The molar mass of a given substance is defined as the mass of a sample divided by the moles of that substance in the sample. The units of molar mass follow from its definition.
Besides how do you find the molar mass of an unknown. Mass of 12 C and this number is N A Avogadros constant 6022140857 74 10 23 m o l 1. In other words it is the mass per mole of a substance.
The unit used to measure is grams per mole. Thus Catom 12 amu mol of. 5g 1 mol1703 g.
The SI unit for molar mass is kgmol-1 or kgmol. It is defined as the mass of substance for a given amount. Amu stands for atomic mass units.
Calculate the molar mass of the compound NH42SO4. Keep at least one decimal place in atomic masses from the periodic table. And the Periodic Table conveniently gives the mass associated with N A atoms of each element.
Molar mass of a particular compound can be calculated by adding the molar mass of the elements present in the formula of the compound. Molar mass is the mass of a mole of a particular substance. The molar mass also known as molecular weight is the sum of the total mass in grams of all the atoms that make up a mole of a particular molecule.
Therefore 1 mole of molecules has a molar mass of 16 g. And the Periodic Table conveniently gives the mass associated with N A atoms of each element. The bridge between the particulate and the macroscopic levels is molar mass the mass in grams of one mole of a substance.
How do you find the molar mass of Naoh. Calculate the molar mass of each compound given below. It is the mass in grams of one mole of a molecular compound.
Molecular Mass Definition. Chemistry questions and answers. Calculate the molar mass of the compound NH 4 2 SO 4.
What is in a mole. Molar Mass is a physical property that represents the mass of a substance divided by the amount of that substance. Formula mass is the sum of the masses of atoms present in the empirical formula.
Molecular mass is a number equal to the sum of the atomic masses of the atoms in a molecule. The sum of the mass of the Mg2 ions and the mass of the Cl ions must be equal to the mass of MgCl2. 3007 gmol b 208301 gmol.
Solved Which definition best describes the term molar mass. It is a physical property of substances. The molecular mass gives the mass of a molecule relative to that of the 12 C atom which is taken to have a mass of 12.
The mass of a molecule relative to the mass of a standard atom now 12 C taken as 12000. The molar mass of a compound can be calculated by adding the standard atomic masses in gmol of the constituent atoms. 0 the mass in units of moles O the mass in grams of one mole of a substance O the mass in ounces of one mole of a substance O the mass of one gram of a substance.
To determine the molar mass multiply the atomic mass by the number of atoms in the formula. Therefore the molar mass 159994 gmol. Relative molecular mass Mr is the mass relative to the dalton and has no units.
Molecular mass is a dimensionless quantity but it is given the unit Dalton or atomic mass unit as a means. The mass in gram of one mole of a substance. The molar mass of iron is 55845g per mol.
The units for molar mass are in grams per mole. The molar mass of sodium hydroxide equals 39997 gmol. Solution for 1 Determine the molar mass of MnClO42.
A KBr b Al2S3. Mathematically the defining equation of molar mass is Molar mass massmole gmol. The molar mass is the mass of a given chemical element or chemical compound g divided by the amount of substance mol.
Molar mass of 16 g. Molar mass SI unit is gmol. It can be used to find the number of molecules or atoms but not ions.
What is Molar Mass. What does the term molar mass refer to. Mŏ-lekyū-lăr wāt The sum of the atomic weights of all the atoms constituting a molecule.
Identify the molar masses of H and O.
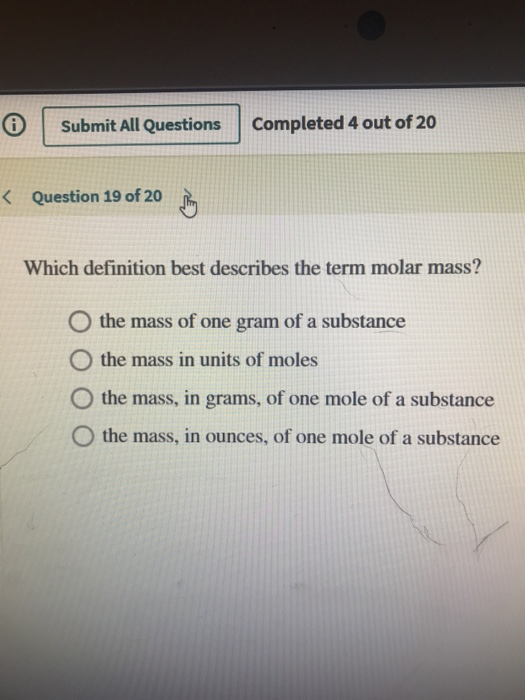
Solved T Submit All Questions Completed 4 Out Of 20 K Chegg Com
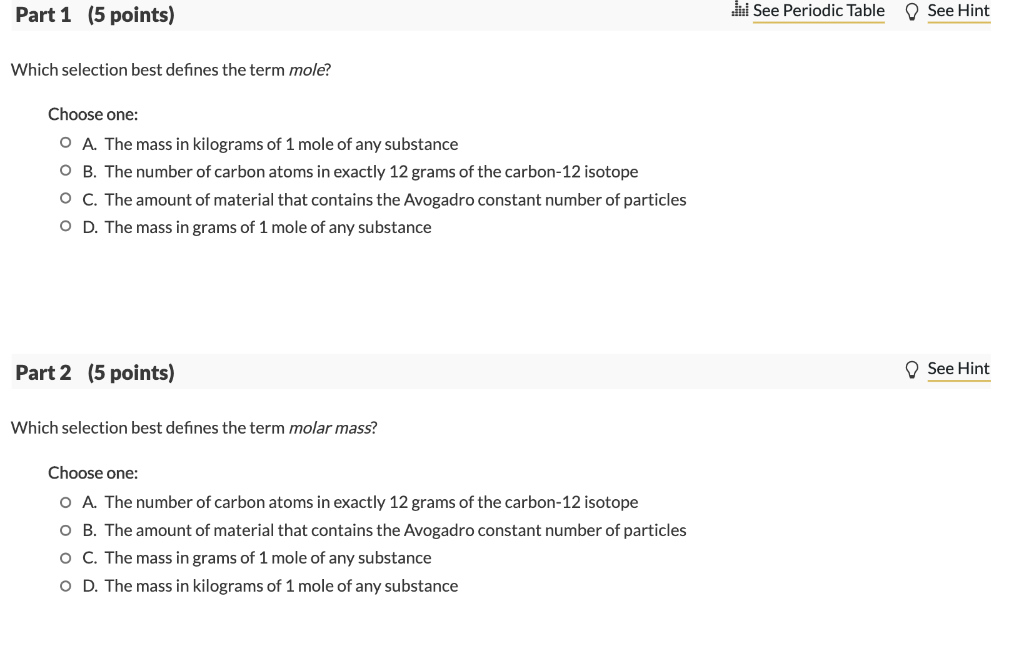
Solved Part 1 5 Points Lui See Periodic Table See Hint Chegg Com

Solved Which Definition Best Describes The Term Molar Mass Chegg Com
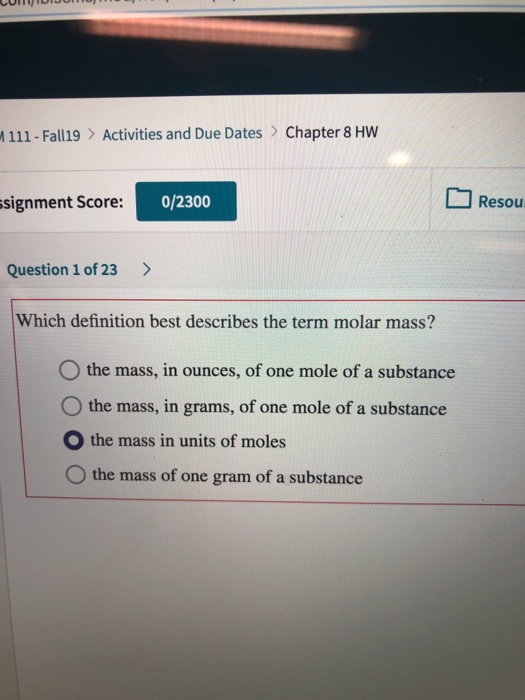
Solved Lumtididun T 111 Fall19 Activities And Due Dates Chegg Com
No comments for "Which Definition Best Describes the Term Molar Mass"
Post a Comment